2018-11-06
In human gut microbiome studies, the logistics of immediately freezing and transporting collected stool samples on dry ice is not always feasible - especially with at-home collections. If the samples are not immediately stabilized, taxonomic profile changes can occur rapidly at room temperature. Furthermore, a challenge with collecting samples from patients exhibiting symptoms of inflammatory bowel disease (IBD) or Crohn’s and chronic colitis, is stool samples can be liquid (Bristol stool type 6 and 7), complicating collection and sample handling, as well as causing a barrier to donor enrolment. To facilitate large cohort studies and produce high quality data, researchers need to be equipped with the most effective tools to easily collect, stabilize and store precious samples.
In partnership with Crohn’s and Colitis Canada, DNA Genotek validated a study protocol using OMNIgene•GUT kits and a specialized collection device to characterize the microbiome profile of IBD patients in remission and active flare. The study illustrates the versatility of the OMNIgene•GUT kits to be used with a unique cohort of patients undergoing dysbiosis; providing a positive easy-to-use collection experience for the donor, and high-quality microbial profiles enabling accurate and reproducible downstream analysis data for the researcher.
Sample collection and compliance rate
Subjects with self-reported IBD were recruited through Crohn’s and Colitis Canada. Naïve donors collected two samples from the same bulk fecal sample using two different collection tools: the standard OMNIgene•GUT spatula and the new spoon accessory (OM-AC2) specifically designed for donors undergoing dysbiosis.
The collected samples in the OMNIgene•GUT kits and the bulk sample were transported to the study coordinator same day as collection, with the bulk sample being transported via the Human Microbiome Project Standard procedure.[2]
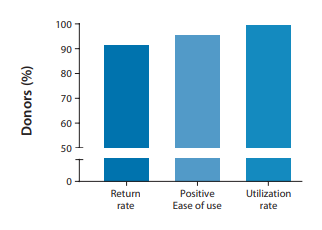
The addition of the spoon accessory allowed donors with all Bristol types (1-7) to easily self-collect a volumetric stool sample into an OMNIgene•GUT collection kit. We’re excited to report that the cohort sample return rate was 92% and all the returned samples were successfully collected. Most notable, 96% of the donors found the collection kits to be easy to use.
Microbiome profiling and neutrality
The DNA yield collected from IBD patients experiencing a flare (Bristol type 6-7) was of high quality and sufficient for downstream sequencing. The total DNA yield from an OMNIgene•GUT sample was 31.43 + 25.67 µg, while the average DNA yield from an OMNIgene•GUT extraction aliquot (0.35mL) was 4.49 + 3.67 µg.
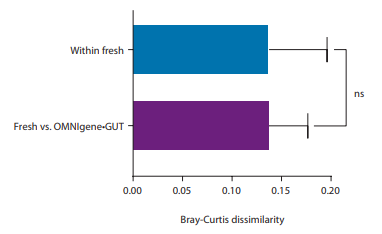
For microbiome studies, it is crucial that collected samples accurately portrait the in vivo profile of the donor. The microbiome profile of the samples collected with OMNIgene•GUT kits in IBD donors experiencing flare was compared to fresh fecal samples. The results clearly illustrates that collection and stabilization of gut microbiome samples with OMNIgene•GUT does not introduce any significant bias in profile recovery.
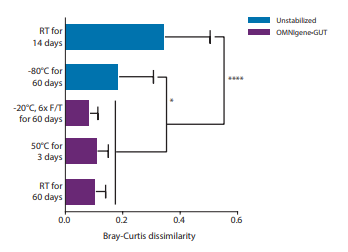
To provide a stabilization solution that researchers can depend on, the OMNIgene•GUT collected samples need to consistently and reproducibly preserve the microbial profile in many different storage and transport scenarios, including stabilization at room temperature for 60 days. OMNIgene•GUT samples were assessed in three different storage conditions and the results were impressive demonstrating that OMNIgene•GUT kits are able to provide microbiome profile neutrality under stringent storage conditions.
Conclusions
The goals of this study were to assess the compatibility and performance of an at-home self-collection method by measuring:
- Donor compliance
- DNA yield and quality
- Microbiome sample stabilization.
The results are clear:
-
OMNIgene•GUT provides donors with an easy-to-use self-collection kit that bolsters study enrolment and sample return rates.
- Versatile collection methodologies of fecal samples using the spatula or spoon accessory provides both volumetric consistency and sufficient high-quality DNA yield for multiple downstream sequencing applications.
-
OMNIgene•GUT samples demonstrated microbiome profile neutrality accurately representing the in vivo donor state.
- Stabilized samples were preserved under harsh simulated shipping conditions and various storage conditions, including room temperature for 60 days.
If you would like more information regarding the OMNIgene•GUT microbiome kits discussed in this article, please contact us at info@dnagenotek.com.
For accessing the full version of this white paper, please click here.
References
[1] Brown A et al. A validated study protocol to compare microbiome and mycobiome profiles of Inflammatory Bowel Disease patients in remission and active flare. DNA Genotek Inc. Poster presented at: Digestive Disease Week (DDW); 2018 Jun 2-5; Washington DC.
[2] Manual of Procedures – Human Microbiome Project (2010).
[3] Brown A et al. OMNIgene®•GUT provides easy self-collection and stabilization of liquid fecal samples for microbiome profiling. DNA Genotek Inc. 2018 Oct 3. White paper accessed 2018 Oct 10. https://www.dnagenotek.com/ROW/pdf/PD-WP-00056.pdf.


