2016-07-14
Collecting fecal samples is not an easy task. Problems can arise during every step of the process, from recruiting donors to analysing the microbial profiles of your samples. Do any of these scenarios sound familiar to you?
- You want to recruit 500 donors for your gut microbiome study, and know that recruitment for this type of research is notoriously challenging. Will it be too difficult to find donors willing to travel to the lab to donate their samples? What if you ask participants to freeze the samples at home and then transport them to the lab on ice? Who wants to store fecal samples in their home freezer, and will they actually do it? Will participants be irritated with the effort required to pack and transport their samples on ice? How will you account for any temperature fluctuations when you analyse the samples?
- You are collecting fecal samples from an indigenous population in a remote area. You have dedicated copious amounts of time and money to acquiring these “once in a lifetime” samples. You would like to ship these samples back to your lab for processing. How long will it take for these samples to arrive? What if they are held at the border for inspection? What happens if the dry ice runs out and the samples are kept at warmer temperatures?
- You have a collection of fecal samples that have been transported to the lab on ice, and then frozen at -80°C. Are these samples reliable? Have the microbial profiles changed during freezing and thawing? Will these samples be difficult to work with? Is there even enough space in the freezer for any more samples?
A great deal of the stress surrounding fecal sample collection results from difficulties with donor compliance, sample storage and transportation. Complications are especially prevalent due to the limited amount of time that an unstabilized fresh sample remains unchanged at ambient temperature and the researcher has to capture an accurate microbial profile. According to Microbial ecologist, Vanessa Hale, at the Mayo Clinic, the gold-standard procedure is to extract DNA from a fresh sample immediately as the microbial composition of a fecal sample starts to shift after one to two days at room temperature[i].
Researchers often resort to freezing their fecal samples to overcome these challenges. However, samples should ideally be frozen at -80°C, and then kept continuously at this temperature until DNA is extracted. This is not practical when collecting from remote areas or from participants who are self-sampling and transporting their samples to the lab. The impact of small changes in temperature has previously been underestimated. As experiments are becoming more specific and minute differences in individual microbiomes are beginning to be revealed, consistent stabilization is becoming indispensable.
What if you could just keep your fecal samples at ambient temperature for 60 days? How would this change your procedures, donor compliance rates and the reliability of your results?
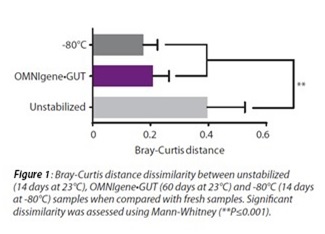 Previously, we demonstrated that OMNIgene•GUT stabilized the microbiome profile in human stool for 14 days[ii]. We are now excited to announce the most current results which show that OMNIgene•GUT can stabilize the microbiome profile in human stool at ambient temperature for 60 days, as well as during real-life shipping and handling conditions and freeze-thaw cycling[iii]. Figure 1 shows no significant difference in the Bray-Curtis distance for the OMNIgene•GUT samples stored at 23°C for 60 days when compared to unstabilized samples stored at -80°C for 14 days. In contrast, the Bray-Curtis distances for the unstabilized samples kept at 23°C for 14 days were significantly different when compared either to the OMNIgene•GUT (60 days) or -80°C storage[iv]. For further information on this you can access the white paper here.
Previously, we demonstrated that OMNIgene•GUT stabilized the microbiome profile in human stool for 14 days[ii]. We are now excited to announce the most current results which show that OMNIgene•GUT can stabilize the microbiome profile in human stool at ambient temperature for 60 days, as well as during real-life shipping and handling conditions and freeze-thaw cycling[iii]. Figure 1 shows no significant difference in the Bray-Curtis distance for the OMNIgene•GUT samples stored at 23°C for 60 days when compared to unstabilized samples stored at -80°C for 14 days. In contrast, the Bray-Curtis distances for the unstabilized samples kept at 23°C for 14 days were significantly different when compared either to the OMNIgene•GUT (60 days) or -80°C storage[iv]. For further information on this you can access the white paper here.
Fecal microbiomes can change drastically in composition over 60 days without proper stabilization, but samples collected with OMNIgene•GUT have been found to be no different than a technical replicate of a fresh sample[v]. Imagine the experiments that are now possible with the ability to take a “snapshot” of microbial DNA at the point of collection and preserve it for 60 days. What could you discover?
References
[i] PLOS ONE, 7:e4695, 2012; Open Microbiol J, 3:40-46, 2009
[ii] Doukhanine, E et al. OMNIgene•GUT stabilizes the microbiome profile at ambient temperature for 14 days and during transport. PD-WP-00042 Issue 2/2015-06.
[iii] Doukhanine, E et al. OMNIgene®•GUT stabilizes the microbiome profile at ambient temperature for 60 days and during transport. www.dnagenotek.com PD-WP-00042/2016-05.
[iv] Doukhanine, E et al. OMNIgene®•GUT stabilizes the microbiome profile at ambient temperature for 60 days and during transport. www.dnagenotek.com PD-WP-00042/2016-05.
[v] Song, SJ et al. (2016). Preservation Methods Differ in Fecal Microbiome Stability, Affecting Suitability for Field Studies. mSystems 1.3. e00021-16.


