2016-09-13
Standardization of microbiome protocols is an increasing hot topic. It was recently discussed at a National Institute for Standards and Technology workshop and is required to make large scale microbiome research efforts like the White Houses’ National Microbiome Initiative possible. The only thing that is potentially discussed as often as standardization is the “inevitable” jump from using 16S rRNA sequencing to query microbiome profiles to a whole genome sequencing (WGS) based approach. In a recent publication in Nature’s Scientific Reports entitled “A robust ambient temperature collection and stabilization strategy: Enabling worldwide functional studies of the human microbiome”, Dr. Ericka Anderson along with colleagues and collaborators from HLI and JCVI tackle both of these topics as part of the HLI program to bring standardization to their microbiome sequencing process.
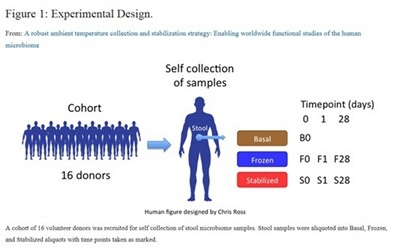 In this study, stool microbiome samples from 16 adult volunteers were collected and divided into three major treatments: Basal: freshly extracted without stabilization or storage which served as the reference or ground truth sample, Frozen: storage at ‐ 20°C and Stabilized: stabilized and stored in OMNIgene•GUT kit (OMR-200/OM-200)*. The authors noted that they wanted to emphasize conditions that were more readily available globally, which is likely why a ‐80°C condition was not included. For all three treatments, DNA was extracted at day 0 and for the frozen and stabilized samples DNA was extracted after 1 and 28 days. Samples were processed and analyzed using HLI’s proprietary microbiome pipeline.
In this study, stool microbiome samples from 16 adult volunteers were collected and divided into three major treatments: Basal: freshly extracted without stabilization or storage which served as the reference or ground truth sample, Frozen: storage at ‐ 20°C and Stabilized: stabilized and stored in OMNIgene•GUT kit (OMR-200/OM-200)*. The authors noted that they wanted to emphasize conditions that were more readily available globally, which is likely why a ‐80°C condition was not included. For all three treatments, DNA was extracted at day 0 and for the frozen and stabilized samples DNA was extracted after 1 and 28 days. Samples were processed and analyzed using HLI’s proprietary microbiome pipeline.
The authors found that OMNIgene•GUT samples returned higher nucleic acid output per mg stool input and that OMNIgene•GUT samples were equivalent to the paired fresh or frozen samples when comparing sequencing metrics, relative abundance of the top 30 species or of the 3 most abundant phyla (Bacteroidetes, Firmicutes and Actinobacteria), functional profiles (as assessed by WGS) and diversity measures.
Based on their results, and the additional functionality provided by OMNIgene•GUT (namely ambient temperature storage, immediate stabilization and homogenization of the sample, reproducibility of aliquots and reduction of freeze/thaw cycles), the authors state that
“global implementation of the Genotek kit, or a similar collection/stabilization device, would present the microbiome community with increased consistency and standardization of sample collection”.
Anderson concludes that
“collection and stabilization of stool microbiome samples with the DNA Genotek collection device, combined with our extraction and WGS, provides a robust, reproducible workflow that enables standardized global collection, storage, and analysis of stool for microbiome studies.”
So what are your thoughts on the recent focus on standardization of microbiome studies? And what do you think about the inevitable shift away from 16S rRNA sequencing towards WGS? We would love to hear your thoughts in the comments section below. If you’d like more information on microbiome-optimized sampling kits, click below.
*OMR-‐200 is available for sale in the USA, and is intended for research use only, not for use in diagnostic procedures. OM-‐200 is available for sale outside the USA and is CE marked for in vitro diagnostic use.


