2023-11-14
 This year marked an important milestone for the annual American Society of Human Genetics (ASHG) conference—the largest global meeting of human genetics researchers and experts, from 77 countries this year—as the Society celebrated its 75th anniversary. The ASHG scientific conference included invited speaker sessions, platform discussions, poster presentations , and more. ASHG also held its annual exhibitor conference, showcasing biotechnology vendors and industry experts. Outdoing the previous year, a total of 8,289 people registered for the 5-day event including 1,443 exhibitors.
This year marked an important milestone for the annual American Society of Human Genetics (ASHG) conference—the largest global meeting of human genetics researchers and experts, from 77 countries this year—as the Society celebrated its 75th anniversary. The ASHG scientific conference included invited speaker sessions, platform discussions, poster presentations , and more. ASHG also held its annual exhibitor conference, showcasing biotechnology vendors and industry experts. Outdoing the previous year, a total of 8,289 people registered for the 5-day event including 1,443 exhibitors.
ASHG took place at the Walter E. Convention Center in the U.S. capital of Washington, D.C. from November 1-5. DNA Genotek™ was elated to be able to meet with attendees on site to discuss ongoing human genetics research and breakthroughs. We were fortunate to have one of our own, 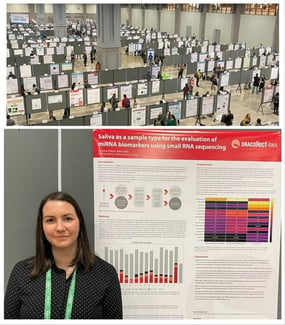 Christina Dillane (Technical Applications Scientist III from the Research and Development team), present an accepted poster showcasing our findings on using saliva as a sample type to evaluate miRNA biomarkers. Samples in the research leveraged DNA Genotek’s ORAcollect™•RNA saliva collection device.
Christina Dillane (Technical Applications Scientist III from the Research and Development team), present an accepted poster showcasing our findings on using saliva as a sample type to evaluate miRNA biomarkers. Samples in the research leveraged DNA Genotek’s ORAcollect™•RNA saliva collection device.
For anyone unable to attend, several common themes—including the value of structural variants, incorporating multi-omics, and leveraging RNA-based data—were highlighted at the conference in specific sessions such as advancing diagnoses for rare genetic diseases, going beyond DNA sequencing to identify disease causes, and CNVs in large-scale studies. As we look back at the conference, we reflect on a subset of these shared themes, summarized below.
Why do structural variants matter?
This year, several oral presentations at ASHG discussed the value in identifying and characterizing structural variants (SVs). Due to their larger size and potential impact on gene function, SVs typically offer a greater wealth of information than the smaller single nucleotide polymorphisms (SNPs), sometimes referred to as single nucleotide variants (SNVs).
One presentation looked at genetic variation from long-read sequencing in a cohort of >1000 African-Americans from the All of Us research program. These researchers found that SVs often replace SNVs as lead markers associated with a genome-wide association study signal and that large SVs were ~50-fold more likely to affect gene expression than SNVs.
The detection of SVs was also a critical point raised in several presentations; in the research discussed above, short-read NGS was found to miss 75% of SVs, while long reads enabled more comprehensive SV discovery. This suggests, as expected, that long reads are better for identifying SVs than short-read technologies.
In an oral presentation analyzing copy number and SV association with cancer pre-disposition from a pan-cancer cohort from the 100,000 Genomes Project, large variants were found to be rare compared to the average number of SNPs per genome. Interestingly, despite their rarity, large variants accounted for a large proportion of inter-individual variability (0.1% genetic difference between two people when only considering SNPs, but this increased to 1.5% when you factored in large variants).
|
The study of SVs, best detected using long-read sequencing approaches, can offer a broader understanding of genomic variation and its role in human health than studying SNPs alone. |
Incorporating multi-omics into your workflow
 Multiple presentations and sessions—including an industry education session about harnessing the power of multi-omics from a single sample—highlighted the immense utility of multi-omics in advancing our understanding of human health. Multi-omics, also known as integrated omics, pan-omics, and trans-omics, aims to combine two or more omics datasets to aid in data analysis, interpretation, and visualization to determine the mechanism of a biological process.
Multiple presentations and sessions—including an industry education session about harnessing the power of multi-omics from a single sample—highlighted the immense utility of multi-omics in advancing our understanding of human health. Multi-omics, also known as integrated omics, pan-omics, and trans-omics, aims to combine two or more omics datasets to aid in data analysis, interpretation, and visualization to determine the mechanism of a biological process.
This holistic approach to study enables a deeper exploration of the genetic basis of diseases, identification of novel biomarkers, and understanding of biological pathways and networks. By combining multiple layers of omics data, researchers can unravel intricate disease mechanisms, facilitate personalized medicine, and ultimately improve patient outcomes. Incorporating multi-omics into a research workflow offers several significant benefits:
-
Allows for a more comprehensive understanding of biological systems by analyzing multiple layers of biological information simultaneously, enabling researchers to uncover complex interactions between different molecules, pathways, and cellular processes.
-
Can enhance accuracy and reliability by integrating different omics datasets, which reduces the likelihood of biased or incomplete results.
-
Enables the identification of potential biomarkers, therapeutic targets, and personalized medicine strategies, as it provides a deeper insight into disease mechanisms and individual variations. In addition, having multiple biomarkers allows for more specific drug targets.
-
Can support hypothesis generation, data mining, and the discovery of novel associations, facilitating breakthroughs in scientific research.
One of the presentations at ASHG described a multi-omic study utilizing genomics and metabolomics to predict 12 common diseases in 500,000 individuals from three biobanks, including the U.K. biobank. Metabolic biomarkers were found to associate with nearly all 12 common diseases, and the addition of metabolomic data was deemed particularly valuable as metabolomic biomarkers are “closer” to the disease process compared to genetic information. Ultimately, existing disease prediction tools can be improved with the integration of both genomic and metabolomic biomarkers and risk scores.
| Multi-omics is on the rise as researchers continue to observe the benefits of a more integrative and holistic approach to diagnosing and studying various diseases. |
Leveraging RNA-based data
RNA, the bridge that connects genes and proteins, can offer functional insights into cellular processes, complementing genomic and proteomic datasets. RNA sequencing technologies enable the generation of high-resolution transcriptomic data that is valuable for studying disease mechanisms and identifying potential therapeutic targets. From their research, many researchers at ASHG showcased how they leveraged RNA data with several speaking about how they improved molecular diagnosis and testing for various diseases.
One presentation touched on the emergence of RNA-Seq as a powerful tool for molecular diagnosis using patient-derived tissues/cells. In this presentation, the clinical utility of Deep-RNA-Seq (sequencing the human transcriptome at ultra-high depths) in Mendelian disorders was discussed. In another presentation, the analysis of RNA-Seq data from over 5,000 individuals in the 100,000 Genomes Project identified new potential diagnoses for patients with rare diseases. A third presentation went over how transcriptome-based data aided in their molecular diagnosis of unknown infantile epileptic spasms syndrome—a common early childhood developmental epileptic encephalopathy (DEE). Combining transcriptome outliers with whole exome or whole genome sequencing seemed to improve molecular diagnosis rates for infantile epileptic spasms.
RNA-Seq was brought up again with respect to germline cancer predisposition testing. In a cohort of 43,000 individuals undergoing hereditary cancer DNA testing, researchers shared their findings at ASHG that adding RNA-Seq data to testing simultaneously increased the positive yield while decreasing the inconclusive rate of multi-gene panel testing.
| Leveraging transcriptomic data seems to improve molecular diagnosis and testing for a wide range of genetic diseases. |
For those interested in complementing their DNA-based datasets with RNA-based data, DNA Genotek™ has recently launched our all-in-one OMNIgene™•SALIVA DNA and RNA collection device. Capable of total nucleic acid collection from humans and microbes, this sample collection device enables multi-omic research and provides convenience and reliability, allowing for easy, non-invasive collection of saliva samples. Designed to stabilize both DNA and RNA at room temperature, the device preserves samples and ensures accurate and reliable results for a variety of applications. The OMNIgene™•SALIVA DNA and RNA collection device provides a streamlined solution for convenient and efficient sample collection without the need for costly cold storage and transportation requirements.

DNA Genotek™ is proud to offer products that support clinical and/or research for human genomics. If you are interested in exploring or expanding your DNA and RNA sample collection abilities, learn more about our latest product, the OMNIgene™•SALIVA DNA and RNA collection device on our website or send us an email at info@dnagenotek.com.
To request a free sample of the OMNIgene™•SALIVA DNA and RNA device, please click here. For other collection devices, please click the image below: