2023-02-10
With the advent of the new year, DNA Genotek was pleased to attend the 8th annual Festival of Genomics (FOG) conference in-person in London, U.K., from January 25-26, 2023. This year, FOG focused on multiple important topics within the genomics field including advances in sequencing approaches, clinical genomics, cancer genomics, single-cell and spatial analysis, liquid biopsies, integrating biodata into a workflow, and multi-omics. Below, we discuss some of the big picture takeaways from our attendance at this conference.
Multi-omics – driving translational medicine
A major theme across several talks at FOG was translational medicine, and how we can move from basic biology towards clinical utility, ultimately leading the charge towards precision medicine. In this context, many experts discussed the importance of multi-omics and taking a systems biology approach where genetic, epigenetic, transcriptional and post-translational events are considered as we attempt to design studies, encourage discovery and integrate large datasets to inform decisions related to precision health.
Importantly, for the oncology field – a focus of the research and trials discussed at FOG – it is necessary to integrate data in a multi-modal fashion. With cancer patient samples being sequenced at scale, the rapid growth of multi-omic data generation, paired with frequent radiological and histological imaging, the challenge is no longer a lack of data, but rather effective and meaningful data-integration. Multi-modal integration of these diverse datasets presents the opportunity to advance precision oncology far beyond the standard genomic and molecular approaches.
As many shared their insights and work to support this challenge, a Microsoft sponsored session highlighted exciting new advances in this area to drive towards open-access cloud-based technologies to enable the use of multi-modal datasets. Some noteworthy highlights from this session included:
-
Microsoft Genomics – powered by Azure, this platform is designed to provide reliable, secure and global data storage enabling efficient management of genomic analysis pipelines and data manipulation.
-
Terra by The BROAD Institute – a cloud-native platform for biomedical researchers to access data, run analysis tools and to collaborate.
-
Zetta Genomics – a genomic-native tertiary analysis tool, built on an open-source platform, that integrates clinical metadata and genomic information in real-time, and aims to enable insights at scale and speed.
Together, such platforms will allow for the multi-modal analysis of complex datasets at scale, which will ultimately lead to the advancement of research, discovery and translational medicine.
From sample type to analyte
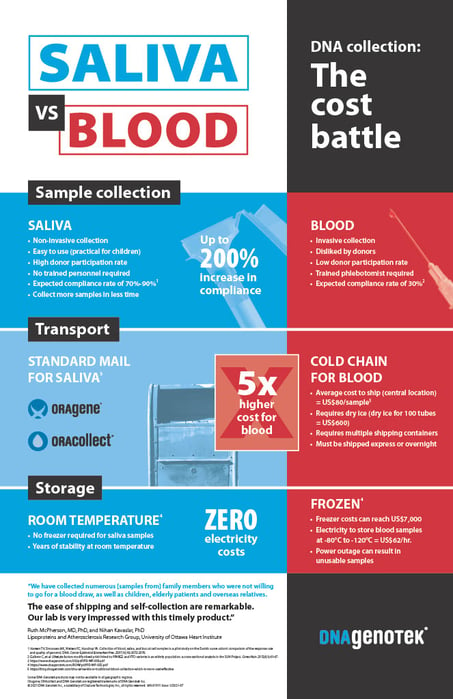
Sample type and patient access were topics discussed across several sessions, as a key determinant to enabling robust multi-omic data generation, as well as their impact on health outcomes and economics. Traditionally, blood has been utilized as a sample type to acquire cellular DNA and RNA for genomic and transcriptomic sequencing, respectively; however, saliva as a sample type has been shown to have several advantages over blood, detailed in-depth here and here.
Liquid biopsy was a buzzword at FOG, with the ultimate goal of incorporating liquid biopsies into routine cancer screening and monitoring programs. Exciting work on cell-free circulating tumour DNA (ctDNA) was shared from Dr. Jacqueline Shaw, a Professor of Translational Cancer Genetics at the University of Leicester. Some of her work highlighted the utility of patient-specific ctDNA analysis from blood as a liquid biopsy to monitor breast cancer recurrence following primary treatment. This work highlights a window of opportunity where these patients may be candidates for subsequent therapeutic intervention. There are, however, challenges associated with using ctDNA in early cancer detection, and there is a need for multi-analyte analysis including cell-free DNA, epigenetic markers and extracellular vesicle RNA to inform diagnostic and therapeutic decisions.
During the panel discussion – “Pushing Liquid Biopsy into Routine Diagnostics,” urine as a liquid biopsy was at the forefront of the discussion. There is growing evidence on the utility of urine as a liquid biopsy for urogenital cancers and other types, including breast and lung cancers. Although there is still a need for research in this area, with a heavy focus on technology advancement, the panelists agreed that urine has the potential to be the future of liquid biopsy in routine diagnostic care. Our sister company, Novosanis, offers Colli-Pee™, a patented first-void urine self-collection device. The Colli-Pee™ device is uniquely positioned to enable volumetric collection and preservation of first-void urine to support ongoing discovery and diagnostic research in the oncology field.
There was also focus on the health economic considerations within this space, and the potential for decreasing healthcare costs associated by routine tissue biopsy, as well as reducing foot traffic in healthcare settings by enabling future at-home sampling and/or testing platforms.
Harnessing real-world evidence and population health data – living in a post-pandemic world
Due to the COVID-19 pandemic, there was an accelerated change in the genomic and infectious disease fields. Globally, several countries coordinated a rapid public health response through the expansion of existing sequencing infrastructure to support variant screening measures and population surveillance. Within this, the field of genomics was positioned to:
-
Generate data at scale
-
Inform public health policy
-
Enable the interlinkage of data at a global level
Because of these changes, as a global society, we are now better positioned to incorporate pathogen genomic surveillance programs to increase our future pandemic preparedness. Importantly, the COVID-19 pandemic highlighted the need for this level of collaboration across borders, bringing research communities to the forefront of the post-pandemic reality. It was obvious at FOG that academic and commercial entities were empowered to leverage these learnings to power the future of discovery, diagnostics and therapeutics.
In addition, population health, the use of real-world data (RWD) and real-world evidence (RWE) were woven into the discussions across several sessions. The COVID-19 pandemic now serves as a case study for how RWD and RWE can advance and inform public health and policy decision making, expanding our capacity and efficiency to conduct post-market studies to evaluate the safety and effectiveness of future diagnostics and therapeutics.
Several challenges must be addressed before we can unlock the true potential of integrating RWD. Some of the hurdles discussed include a lack of standardization in healthcare records, bias due to underrepresentation of specific populations and accessibility to data. There is a need for initiatives to address these challenges, however, the potential of leveraging RWD and RWE to supplement decision-making throughout a product lifecycle is invaluable. An example provided at FOG that demonstrated the application of RWE, was during pre-clinical drug development for rare diseases. Harnessing the power of RWE enables drug developers to access historically difficult patient pools at scale and provides potential health solutions for those suffering from a rare disease.
Concluding remarks
Several big picture takeaways were discussed in sessions at the FOG conference. First, the shift in basic research to utilizing multi-omics to study diseases is driving translational medicine advancement, thereby allowing us to come closer to a future where precision health and medicine may be incorporated into our healthcare systems. In this context, at FOG, oncology research was highlighted in several presentations along with the need for appropriate data integration tools to integrate multiple “omic” datasets. Cancer was also a frequent discussion point in the context of sample types and analytes, including the move towards liquid biopsies for cancer screening. Finally, the last major theme was the COVID-19 pandemic's impact on the use of RWD and RWE and how it served as a catalyst in genomics and infectious disease.
If you are interested in expanding your DNA or RNA sample collection abilities and diversifying your cohort population, learn more about our Oragene™•DNA, ORAcollect™•DNA, and ORAcollect™•RNA products on our website or send us an email at info@dnagenotek.com. Click on the graphic below to test out our kits for yourself!


