2021-07-16
After more than a year into the COVID-19 pandemic, the demand for returning to in-person learning in K-12 schools is growing. Virtual/hybrid education has had a significant impact on student's academic achievement, food security, and mental and physical health.[1]The loss of in-person learning has particularly affected underserved and low-income populations, which are less likely to have the resources to facilitate virtual learning and more likely to depend on school meal programs for nutrition.[1],[2]
As reopening strategies are being developed, COVID-19 prevention methods and baseline monitoring must be included. Alongside on-site hygiene measures and increased vaccination coverage, regular testing for COVID-19 plays an integral role in creating safe environments for educators, students, and families.[2] As part of President Biden’s National Strategy for the COVID-19 Response and Pandemic Preparedness, the U.S. Department of Health and Human Services (HHS) will provide US$10 billion to states to support and increase COVID-19 screening tests to enable schools to reopen in a safe way:[3]
Weekly testing of all students, teachers, and staff can reduce in-school infections by an estimated 50 percent.[4]
Regular testing in schools can reduce outbreaks, increase confidence among families and staff and ensure all communities (rural and urban) have access to testing.[5]
COVID-19 testing methods and sample types
Molecular tests and antigen tests are the two options to detect active COVID-19 infection. These tests can vary in type of analysis, degree of sensitivity, and time required for results. The sample types that can be collected for COVID-19 detection include nasopharyngeal swab, anterior nasal swab, throat swab, and saliva.[5]
| Testing method | Type of Analysis | Sensitivity | Analysis location | Time required for results |
| Molecular test (e.g., RT-PCR) | Detects genetic material from the virus | Accurate | Generally off-site or in a lab | Hours/days |
| Antigen test | Detects specific proteins on the virus | Less accurate | Generally on-site | Minutes |
When deciding which test to use for COVID-19 testing in schools, reliability, feasibility, and time required for results must be considered. Additionally, choosing a sample type that is easy to collect, especially when students are young children, is important to keep in mind.
Sample collection efforts initially focused on nasopharyngeal (NP) swabs. Unfortunately, this procedure can be uncomfortable and invasive[6] and requires test administrators to come into close contact with donors, increasing the risk of spreading the virus.[7] Use of anterior nasal (AN) swabs, which are a less invasive swab of the nostril, is currently one of the standard procedures for COVID-19 testing.[5] AN sampling has shown similar sensitivity to NP sampling in detecting COVID-19, but it too can require supervision.[8] Repeated use of any form of nasal swabs can be stressful for children and may require some supervision, which can contribute to lower uptake and lead to challenges for parents, caregivers, and teachers.
An article in the New York Times highlighted the experience of one family whose child had to receive a nasal swab:[9]
A visit to the hospital didn’t freak Zoey out — she was a sturdy patient who was accustomed to medical procedures. But after Jaeger told her daughter she had to get a coronavirus nasal test when they got to the hospital, Zoey refused to get in the minivan. Jaeger coaxed her into going, and ultimately had to restrain her daughter in her lap while a nurse reached into the van and swabbed Zoey’s nose. “It was such a nightmare. I was crying, Zoey was crying. It was just a mess, there’s got to be a better way,” Jaeger said. “She was traumatized.”
Using saliva as a sample type for COVID-19 testing in schools
In 2020, sample collection expanded to include saliva as a sample type for COVID-19 testing. Saliva has been shown to accurately detect COVID-19 in early infections, offering similar sensitivity to other sample types.[10]
Saliva also allows for easy, painless, non-invasive self-collection, with little or no supervision. This feature minimizes or eliminates exposure to COVID-19 for test administrators and the need for personal protective equipment, making it an attractive and cost-effective option for large-scale testing.[7],[10]
DNA Genotek® saliva collection devices support COVID-19 diagnostic efforts for return to in-person learning. OMNIgene®•ORAL (spit-based collection) and ORAcollect®•RNA (oral sponge collection) are easy to use and feature a non-toxic chemistry that inactivates SARS-CoV-2. The devices are authorized for use under Emergency Use Authorization (EUA) to collect, stabilize and transport saliva samples for COVID-19 testing.[11]
The SUNY experience
Using saliva as a sample type for COVID-19 screening for return to in-person learning has been demonstrated in several real-world examples.
Starting in late 2020, SUNY colleges and universities in New York state implemented a COVID-19 testing program for students, faculty, and staff. The aim was to allow SUNY institutions to return to in-person learning in a safe way by identifying and/or containing the virus as quickly as possible. The methodology, developed and validated by SUNY Upstate Medical University and Quadrant Biosciences, uses DNA Genotek’s ORAcollect®•RNA device for saliva sample collection.[12]
Thanks to Upstate Medical’s world-leading saliva test, SUNY has been at the forefront of monitoring and containing this virus on college campuses. [Upstate and Quadrant’s] new, state- of-the-art testing lab further expands our testing capacity and shortens the turnaround time for results — providing further assurance that we can protect students, faculty, staff and our surrounding communities from COVID until it’s in the rearview for good.[13] - James Malatras, SUNY Chancellor
(I) How does the process work?
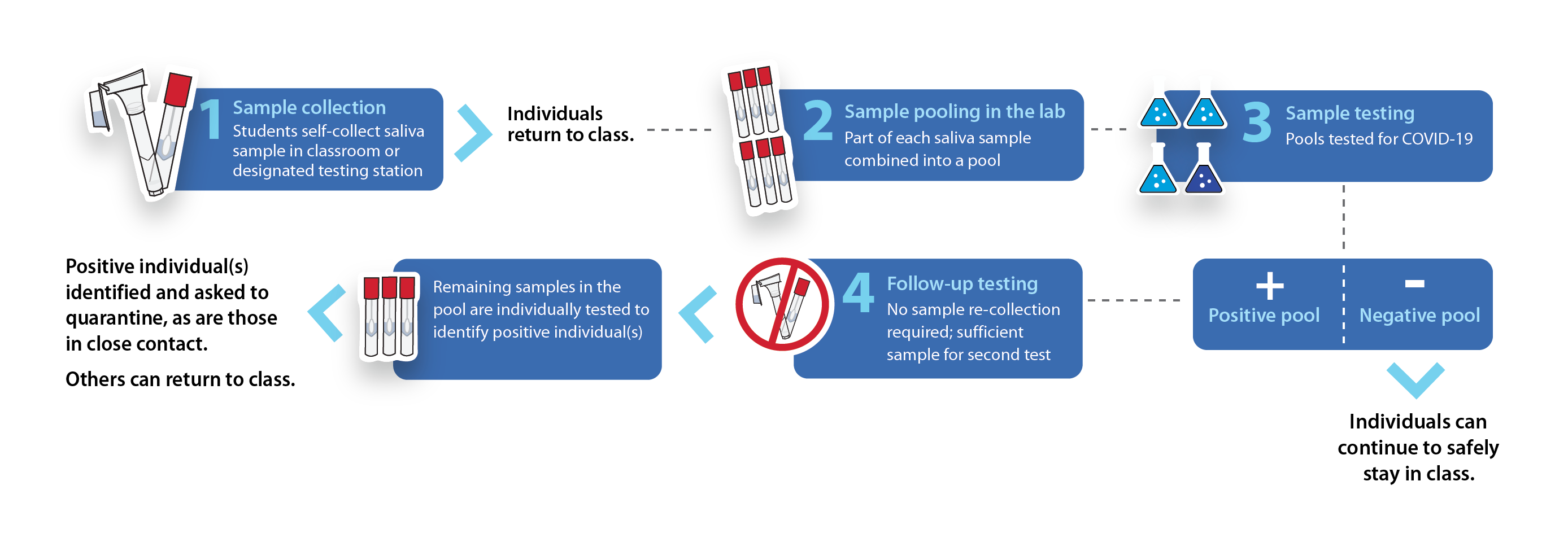
To allow for the extensive and frequent testing required across all campuses, the methodology used pooled testing — an innovative approach that analyzes multiple samples simultaneously.
Once samples are collected with the ORAcollect®•RNA device, they are grouped together in batches to form a pool of about 12 individuals.[12] The samples are then tested using Quadrant-SUNY’s Clarifi COVID-19 assay.[14] A positive test means that at least one individual in the pool is positive and that each saliva sample needs to be tested again to find the exact positive case(s).
A pooled testing approach using saliva reduces the risk of contamination, as samples are collected individually on-site and pooled in the lab. Additionally, because analysis is performed in the lab, sample re-collection is not required if a pool tests positive, allowing a higher number of individuals to be tested at one time. The process is quicker and requires fewer testing resources than nasal sample collection.[12]
School environments are well-suited to use pooled testing because of their current low positivity rate and the natural groupings or cohorts they provide. This means all students in a particular class would be expected to quarantine if a group or a classmate tests positive.[2],[5]
COVID-19 school testing pooled saliva workflow
(II) What was the feedback from users?
Users preferred saliva sampling through the ORAcollect®•RNA device to other collection methods:
You have eliminated the stress of covid testing for so many children. The NP (nasopharyngeal) swabs are traumatic for most kids and frequently puts the physician in the difficult position of getting close to a sick child and having them cough and sputter in front of you; so you have made it safer for me to administer these tests. It is not only an improvement in comfort. I have had more kids than I can count come in to get tested BECAUSE it was the oral swab and not the NP one. Their threshold for testing has reduced, thereby improving our ability to identify spread. The positive ripple effects of your work are palpable in my daily work and I want to thank you from the bottom of my heart.[13]
Alison McCrone, MD, FAAP Assistant Professor of Emergency Medicine and Pediatrics, Medical Director of Upstate Golisano After Hours Care
Additionally, children in schools in New York state describe the Clarifi COVID-19 saliva collection process as easy, soft, and painless and stated their preference for this test over nasal swabs.[15]
(III) What is the accuracy of saliva samples using the Clarifi COVID-19 test?
Test sensitivity is an important consideration when evaluating testing kits. Poor sensitivity can result in false negatives, increasing the spread of the virus. The Clarifi COVID-19 saliva-based test, authorized for use under Emergency Use Authorization (EUA), is among the most sensitive available.[16]
A scalable future for COVID-19 detection using saliva
The SUNY example highlights how COVID-19 screening with saliva pooled sampling can be an effective way to return to in-person learning and is an approach that can be extended to K-12 schools.
DNA Genotek’s saliva collection devices enable non-invasive self-collection of saliva with minimum supervision. The kits are compatible with most sample processing and analysis techniques. Our kits are being used for symptomatic and asymptomatic individuals in back-to-school, back-to-work, population/community, and travel testing and screening applications.[11]
Contact us to discuss how DNA Genotek can assist with your sample collection requirements at info@dnagenotek.com
References:
[1] K-12 National Testing Action Program (NTAP). https://www.rockefellerfoundation.org/wp-content/uploads/2021/05/K-12-NTAP-Deck-20210419-V104.pdf
[2] Operational Strategy for K-12 Schools through Phased Prevention. https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/operation-strategy.html#anchor_1616080181070
[3] Biden Administration to Invest More Than $12 Billion to Expand COVID-19 Testing. https://www.hhs.gov/about/news/2021/03/17/biden-administration-invest-more-than-12-billion-expand-covid-19-testing.html
[4] COVID-19 Educational Testing. https://covidedtesting.com/
[5] Covid-19 Testing in K-12 Settings: A Playbook for Educators and Leaders. https://www.rockefellerfoundation.org/wp-content/uploads/2021/02/The-RockefellerFoundation-Covid-19-K-12-Testing-Playbook-for-Educators-and-Leaders.pdf
[6] Lee RA, et al. Performance of saliva, oropharyngeal swabs, and nasal swabs for SARS-CoV-2 molecular detection: a systematic review and meta-analysis. doi:10.1128/JCM.02881-20
[7] Saliva samples for COVID-19 testing. https://blog.dnagenotek.com/saliva-samples-for-covid-19-testing-a-scalable-and-practical-way-forward
[8] Nasal (Anterior Nasal) Specimen Collection for SARS-CoV-2 Diagnostic Testing. https://www.cdc.gov/coronavirus/2019-ncov/downloads/OASH-nasal-specimen-collection-fact-sheet.pdf
[9] Coronavirus Tests Can Be Scary for Kids. Here’s How to Make Them Easier.
https://www.nytimes.com/2020/07/15/parenting/kids-covid-19-test.html
[10] Johnson AJ, et al. Saliva testing is accurate for early-stage and presymptomatic COVID-19. https://doi.org/10.1101/2021.03.03.21252830
[11] DNA Genotek’s collection devices for COVID-19 testing. https://dnagenotek.com/US/products/collection-infectious-disease/covid-19-collection-kits/index.html
[12] Chancellor Malatras Announces All SUNY Colleges and Universities to Implement Testing Program to Detect and Monitor COVID-19 Cases on Campuses. https://www.suny.edu/suny-news/press-releases/09-2020/9-4-20-testing/covid-testing-program.html
[13] Clarifi COVID Testing Testimonials.
[14] Clarifi COVID-19 Test Kit Instructions for Use. https://www.quadrantbiosciences.com/wp-content/uploads/2020/11/EUA-Quadrant-clarifi-ifu.pdf
[15] What are the kids saying? https://vimeo.com/566238630/474179ac99
[16] SARS-CoV-2 Reference Panel Comparative Data. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-reference-panel-comparative-data